How is Soap Made and Tested?
Contributed by Jo Gardner
Soap, known to remove SARSCoV-2 from hands in 30 seconds is a particularly useful product, consumed daily, that is under appreciated. According to globalhandwashing.org, “soap helps break down germ-carrying oils. Soap also facilitates rubbing and friction which can remove germs from the hands, so that germs can be rinsed away with water. Using soap also adds to the time spent washing and ensures a more effective wash.”
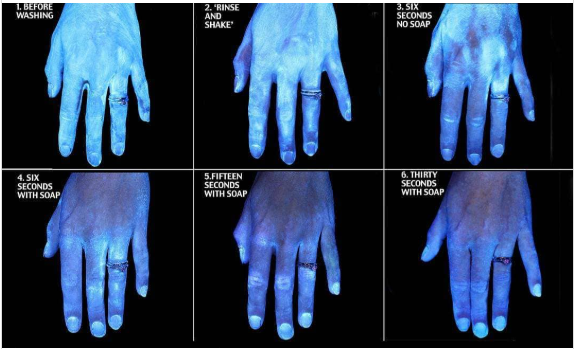
Most of us have seen the illustrations of efficacy of hand washing with only water vs. washing with soap for 30 seconds. Image below posted by Kristenanniebell on Instagram March 3, after receiving it from her mother.
Scented soap has become a cottage industry, requiring only three ingredients to chemically change into soap: oil (this can be animal or vegetable, as long as it is not petroleum based oil), water and lye (sodium hydroxide). Of course, they must be mixed together in correct proportions, to saponify, or turn into soap. The original kettle method of soap production took 4 to 11 days to complete the saponification process. By 1940, manufacturers had found a more efficient process known as continuous process, which needs about 6 hours to complete production of a batch of salt.
Soap Making Kettle Process
In the kettle process the fat, water and alkali were brought to a boil. After which, the fat reacted with alkali to produce soap and glycerin. By adding salt, the glycerin is separated from the soap, which causes soap to rise to the top and glycerin to sink to the bottom of the kettle, where glycerin is then extracted. A caustic solution is added removing small amounts of fat that did not saponify. In the next step, called Strong Change, the ingredients are again brought to a boil so the last of the fat turns to soap. A final boil, called pitching, creates two layers of soap. The top layer, neat soap, is 70% soap and 30% water The lower layer contains impurities such as dirt and salt and water, and is less valuable.
Soap Making Continuous Process
In the continuous process, the steps to soap production include splitting, mixing, cooling and finishing, and milling. In a large vat called a hydrolyzer, the fat is split into fatty acids and glycerin using raised temperature and pressure in a process controlled by pumps and meters. The purified fatty acids are then mixed with alkali to saponify into soap. The soap is either poured into molds, or allowed to harden into a slab, which will be cut into bar sizes. The cooled soap is milled by heavy rollers which crush and knead the soap. The glycerin which was removed in the beginning is used to manufacture hand lotion, drugs and nitroglycerin.
The Chemistry of Soap
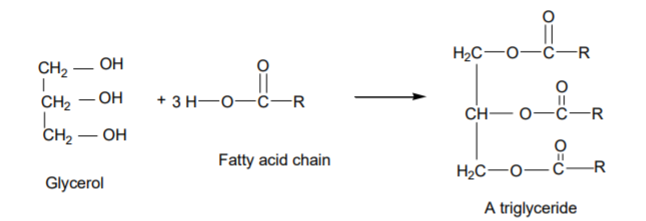
In Soap making Triglycerides (fats or oils) are hydrolyzed usually by mixing with sodium hydroxide in a process of saponification in a reaction seen in the image below.
When producing a specific brand of soap today, manufacturers can turn to trusted independent materials testing labs such as NSL Analytical Services to determine the materials’ evaluation and characterization, contaminant identification, chemical analysis, Compositional analysis and material identification, as well as the pH value, the emulsifying power, and the solubility nature of the soap product. Soap, an unsung hero in limiting the spread of SARSCoV-2, can also be scented, hand softening, liquid or bar, and cleansing. And all of those attributes, need to be tested by a lab such as NSL Analytical Services before the product is marketed. If you have a product you are developing, or need regulatory testing on, visit NSL Analytical Services and contact us with your request, or call (877) 560.3943 for information on our accreditation and testing services available.
If you have a question about how a common product is made or tested, email us at social@resourceshark.com or enter your question in the discussion area. And remember, washing your hands for 30 seconds with soap removes germs and viruses.

